Faculty Development Program (e-FDP)
Write-up for Faculty Development Program (e-FDP) on “Restructuring Pharmacy Education for Atmanirbhar Bharat: Time for Transformation”
Lloyd Institute of Management and Technology (Pharm.) was conducted its first Faculty Development Program (e-FDP) on “Restructuring Pharmacy Education for Atmanirbhar Bharat: Time for Transformation” from 20th July, 2020 – 24th July, 2020 that serves a purpose to focus on the need of evolving education pedagogy as per current needs.
It was conducted under the guidance of Dr. Vandana Arora Sethi, Group Director, Lloyd Group of Institutions, and her team. Lloyd was overwhelmed with the 300+ Registration & more than 95 Colleges taking part like DPSRU, Jamia Hamdard, NIPER, Amity university, King Saud University, BIT, KIET, Galgotias University, Guru Jambheswar university, ISF, NIET, Integral University, RKGIT, Sharda University, Taif University, Delhi University, Kashmir University, Vels University and many more. It was scheduled for five days starting from 20th July 2020 to 24th July 2020, at 11:00 am India onwards.
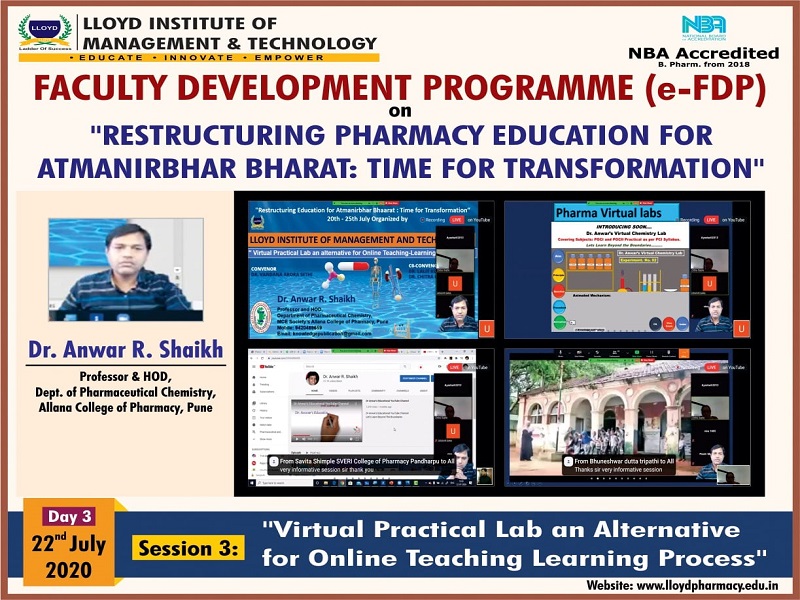
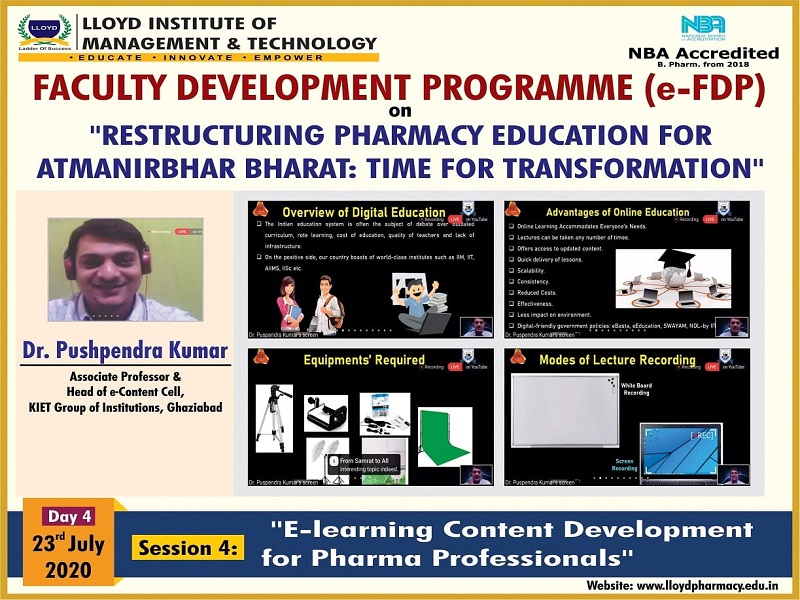
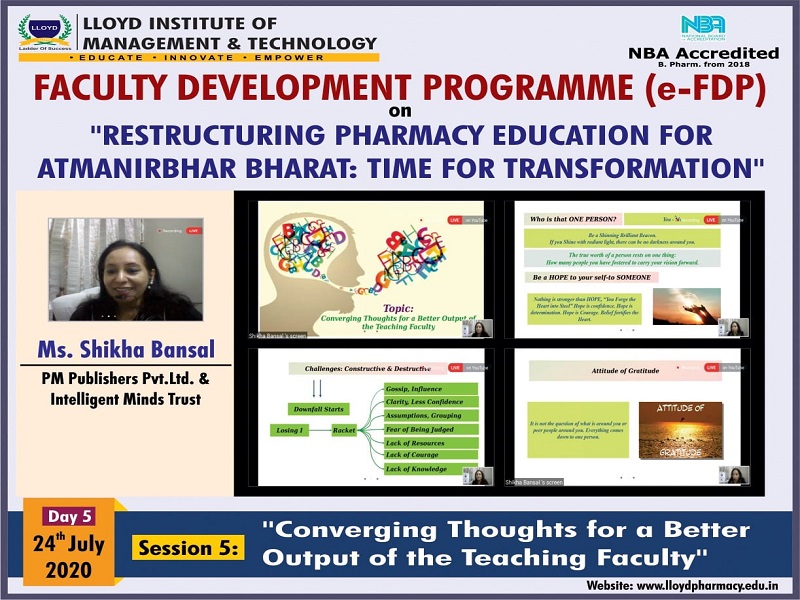
Each day included an insightful session with the magnate speakers with some interesting topics. One the first day Lloyd had Mr. Atul Shirgaonkar, CEO, Insight System Inc., Pune. He spoke on the topic "Good Manufacturing Practices (GMP): Yesterday, Today and Tomorrow". On the second day, Prof. Shubhini A. Saraf, Dean, Dept. of Pharm. Sciences, Babasaheb Bhimrao Ambedkar University, Lucknow, spoke on the topic "3D Printing and it's Relevance in the Post Covid-19 Scenario". The third day included a session titled "Virtual Practical Lab an Alternative for Online Teaching-Learning Process" by Dr. Anwar R. Shaikh, Professor & HOD, Dept. of Pharmaceutical Chemistry, Allana College of Pharmacy, Pune. Fourth day’s session was taken by Dr. Pushpendra Kumar, Associate Professor & Head of e-Content Cell, KIET Group of Institutions, Ghaziabad on the topic, "E-learning Content Development for Pharma Professionals". Finally, Day 5 was concluded with a session titled "Converging Thoughts for a Better Output of the Teaching Faculty" by Ms. Shikha Bansal, Educomp Solutions Pvt. Ltd.
Dr. Vandana Arora inaugurated this session with a warm welcome note and appreciated the indulgence of our delegates, colleagues, and participants across the country. She started her talk with a note on the current scenario of the COVID-19 pandemic and how the studies in the institutions are being shaped and modified accordingly. She projected 4G’s as four-pillars of growth namely “gain more skills, grow your network, get more organized, and lastly become a go-to person for getting things done.” She emphasized that during this COVID era, Education 4.0 is already in, with newer, innovative educational tools already in practice. COVID-19 in disguise has revolutionized the whole education & everyone will emerge more learned & evolved after this.
The Day commenced with the first session as the topic "Good Manufacturing Practices (GMP): Yesterday, Today and Tomorrow" by Mr. Atul Shirgaonkar, CEO, Insight System Inc., Pune. He started the session with the history of GMP and the general concept that was being focused in the early days. He also shared the entire step-by-step process of GMP inspection by FDA and elaborated on a few important terms such as Misbranded drugs, Quality Control, Active Pharmaceutical Ingredients (API), etc. and various guidelines. He elaborated on the term GMP as a Good Manufacturing Practice system that ensures the quality of manufacturing products, like food, drugs, medicines, cosmetics, and pharmaceutical goods that should be consistently produced and controlled according to set quality standards. He discussed GMP and as an applicable tool to all aspects of quality, starting from raw materials to finished dosage forms and encompassing the safety & efficacy of the product. He elaborated on the contents under the GMP like quality management, Sanitation and hygiene, Building and facilities/premises, Equipment, Raw materials, Personnel, Validation and qualification, Handling complaints, Documentation, and recordkeeping, Inspections & quality audits. He even emphasized on Pharmaceutical Quality Systems ICHQ10 Guideline. Besides, he also explained how to sustain GMP Compliance that comprised of the Quality team, Documentation validation, surprise audits, and compliance training. He took his interest and time in answering the follow-up questions from participants.
The second day began with a warm welcome to Prof. Shubhini A. Saraf, Dean, Dept. of Pharm. Sciences, Babasaheb Bhimrao Ambedkar University, Lucknow. She spoke on the topic titled, "3D Printing and it's Relevance in the Post Covid-19 Scenario". She started with the basic introduction of 3D printing and its general idea behind it. She explained her concept by putting forward the basic question on how learners could use this insight of 3D application in this pandemic era. She mainly focused on the benefits of 3D printing in the medical application like customization and personalization. This idea could be used in medical applications like medical.