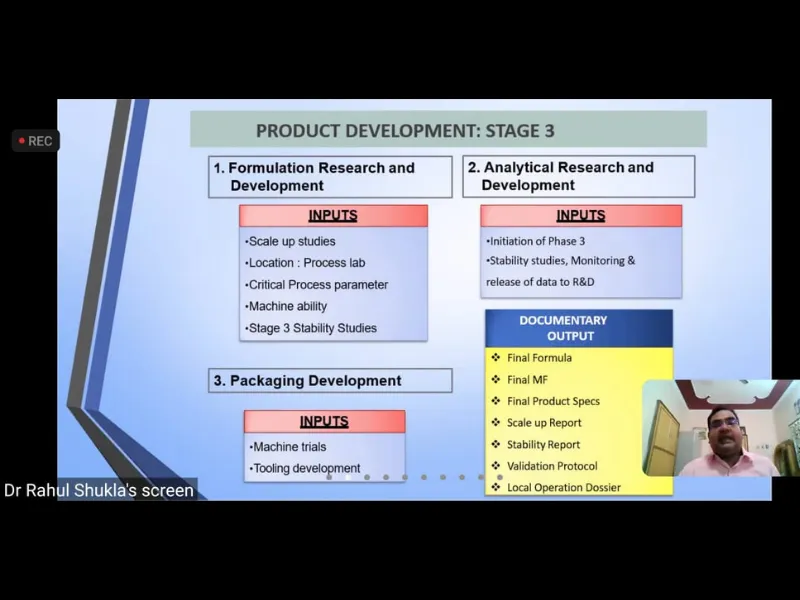
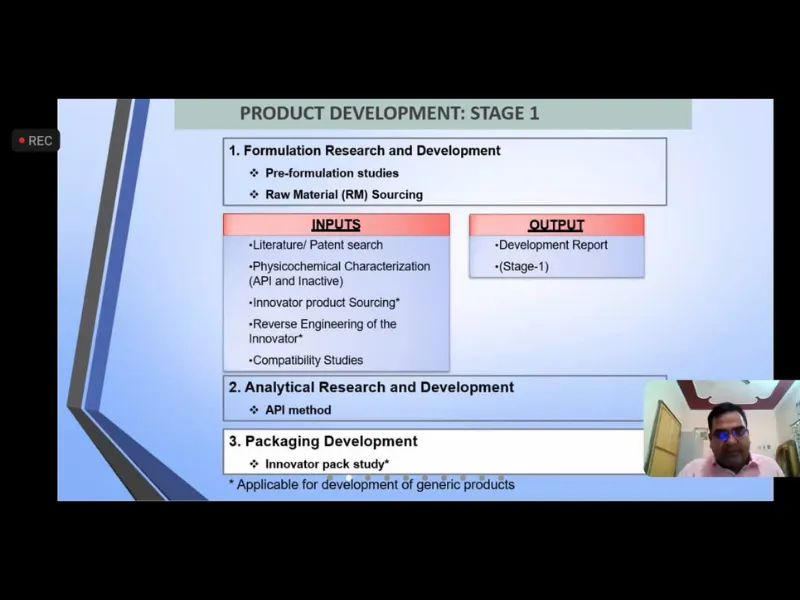
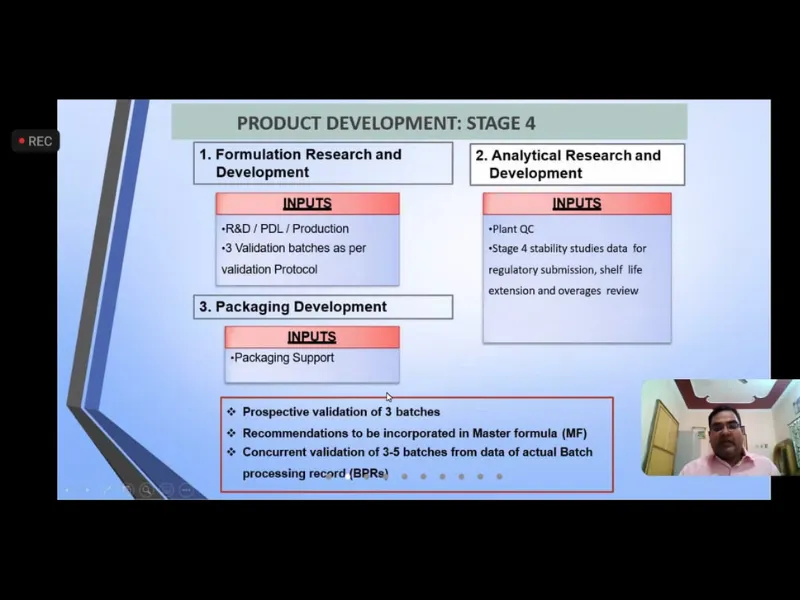
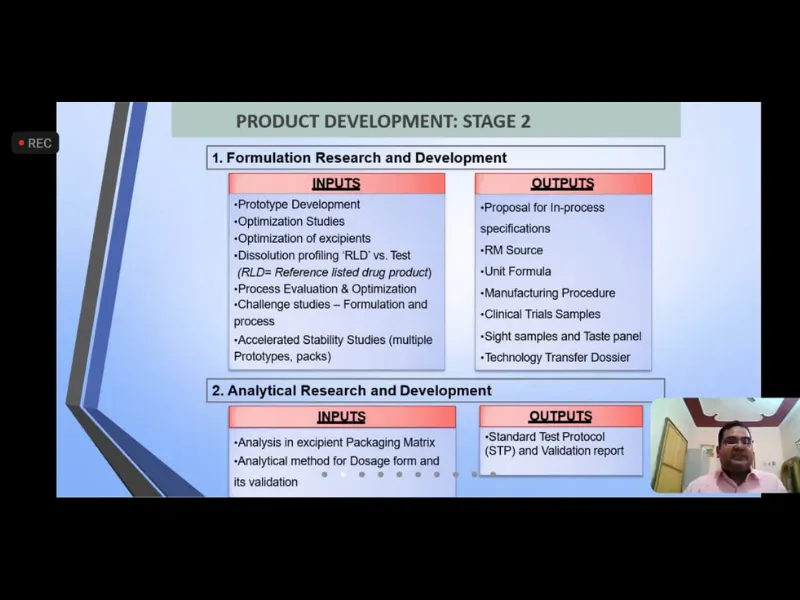
Expert Talk IV-“Drug Development: The journey of a Pharmaceutical from Lab shelf”
Saturday, May 29th 2021: Dr. Rahul shukla Assistant Professor, Department of Pharmaceutics, NIPER, Raebareli gave a guest lecture on: “Drug Development: The journey of a Pharmaceutical from Lab shelf” Once a potential target has been identified, researchers will then search for a molecule or compound that acts on this target. Historically, researchers have looked to natural compounds from plants, fungi or marine animals to provide the basis for these candidate drugs but, increasingly, scientists are using knowledge gained from the study of genetics and proteins to create new molecules using computers. He explained the journey a molecule travels from identification to synthesis. He stated that as many as 10,000 compounds may be considered and whittled down to just 10 to 20 that could theoretically interfere with the disease process. The next stage is to confirm that these molecules have an effect and that they are safe. The process of drug development and marketing authorization is similar across the world. For those drugs that make it to through phase 3, a submission for marketing authorizations is made to the national regulatory authority in most countries.
In the UK, this is the MHRA and, in the US, the Food and Drug Administration (FDA). However, in Europe, drug companies usually now opt to make a central application to the European Medicines Agency (EMA) in order to obtain marketing authorization for the whole of Europe to avoid having to make multiple applications to individual countries. The submission contains preclinical and clinical information obtained during testing, including information about the chemical makeup and manufacturing process, pharmacology and toxicity of the compound, human pharmacokinetics, results of the clinical trials, and proposed labeling. Students cleared their doubts after his talk and were really excited to know many new concepts.